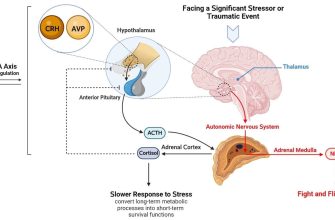
The recent FDA approval of American Generic Cialis offers a promising alternative for individuals seeking erectile dysfunction treatment. This accessible version maintains the same active ingredient, tadalafil, ensuring similar efficacy and safety profiles as the brand-name drug.
Understanding the Benefits of generic options can lead to significant cost savings. Patients can expect lower prices without compromising on quality or effectiveness. With generics entering the market, those previously hesitant due to cost barriers can now pursue treatment more confidently.
It’s essential to consult a healthcare provider before starting any new medication. They can provide personalized advice and determine if the generic Cialis aligns with your health profile and needs. This step ensures not only safe usage but also optimal outcomes.
As you explore treatment options, consider both the availability and the evidence supporting the generic’s reliability. Numerous studies confirm that generic tadalafil performs comparably to its branded counterpart, paving the way for informed decisions regarding your health.
Understanding FDA Approval of American Generic Cialis
The FDA evaluates generic drugs like American generic Cialis through a rigorous process, ensuring safety and efficacy. Generics must demonstrate bioequivalence to the brand-name version, meaning they deliver the same active ingredients at similar absorption rates. This requirement assures doctors and patients of the generic’s reliability.
Key Steps in the Approval Process
The approval process begins with an Abbreviated New Drug Application (ANDA). The manufacturer submits data showing that their version matches the original Cialis in dosage form, strength, route of administration, and intended use. Additionally, the FDA requires stability data to confirm the generic’s shelf-life matches or exceeds that of the brand name.
Compliance and Quality Control
Manufacturers must adhere to strict Good Manufacturing Practices (GMP). The FDA conducts inspections of production facilities to confirm compliance. This commitment guarantees that every batch of generic Cialis maintains the quality and safety standards required by the agency. Once approved, ongoing monitoring helps the FDA enforce these standards, ensuring continuous safety for consumers.
The availability of American generic Cialis fosters affordability and accessibility, allowing more individuals to benefit from treatment for erectile dysfunction. Always consult with a healthcare provider before starting any new medication. They can offer insights tailored to individual health needs and circumstances.
Key Factors of FDA Approval for Generic Cialis in the U.S.
FDA approval for generic Cialis hinges on several critical factors. First, manufacturers must demonstrate bioequivalence to the brand-name version. This means that the generic must have the same active ingredient, dosage form, strength, route of administration, and intended use. It’s essential that the generic produces comparable effects in the body, ensuring patients receive the same therapeutic outcome.
Next, the manufacturing process must meet stringent standards. Facilities must comply with Good Manufacturing Practices (GMP) to ensure product quality, safety, and reliability. This includes adhering to strict procedures and maintaining a clean and controlled environment throughout production.
Clinical data submissions play a major role in the approval process. While generics often do not require extensive clinical trials like their brand-name counterparts, manufacturers must still provide sufficient data to prove safety and efficacy. This may include pharmacokinetic studies and other relevant evidence supporting the generic’s performance.
Additionally, thorough review by the FDA ensures that all labeling and instructions are clear and informative. This transparency helps healthcare providers and patients understand the medication, including potential side effects and interactions.
Understanding these factors can assist stakeholders in navigating the approval process for generic Cialis effectively. Continuous communication with the FDA can also facilitate quicker resolutions to any questions or concerns that arise during the review. By focusing on these key elements, manufacturers can streamline their efforts toward bringing a safe and effective generic version of Cialis to market.
Impact of American Generic Cialis on Accessibility and Affordability
American generic Cialis significantly enhances accessibility for many individuals seeking treatment for erectile dysfunction. The introduction of generic versions permeates the market, driving prices down. This increased competition leads to lower out-of-pocket costs for patients, making it easier for them to obtain necessary medications.
Generic Cialis provides a more affordable option compared to its brand-name counterpart. On average, patients report savings of up to 70% when choosing generics. Such cost reductions are particularly beneficial for those without insurance coverage or those facing high deductibles.
Pharmacies also play a vital role in the distribution of generic Cialis. Increased availability at local and online pharmacies ensures that patients can find the medication conveniently. Many pharmacies now offer price-matching guarantees or discount programs, further enhancing affordability.
Patients can utilize discount cards and patient assistance programs to reduce costs further. These programs often provide additional savings for eligible individuals, making the treatment more accessible to a broader audience.
Education on the availability of generic options is essential. Healthcare providers should actively inform patients about generic Cialis, emphasizing both the affordability and quality. This knowledge empowers patients to make informed choices, ensuring they receive the treatment they need without financial strain.